Coral Reef Acidification
Study the impact of acidity on marine life
- All
- Fun With Food
- Experiments
- Coral

What you’ll need:
Equipment:
- Aluminum foil
- 2 small glass jars or clear drinking glasses
- A pen, marker or pencil
- Tape
- Two small bowls
- Measuring cups
- A chopstick or popsicle stick (optional)
Ingredients:
- 1 egg
- 2 tsp of salt
- 1/2 cup of vinegar
- Water

Activity setup:
- Use tape and a pen to label your glass jars (or drinking glasses). Label the first jar Salt Water and the other jar Vinegar.
- Crack the egg into a bowl. Try to keep the two halves of the shell as intact as possible. Put the egg in the fridge to use later for cooking or baking.
- Add one half of the eggshell to the Salt Water jar and the other half to the Vinegar jar.
- Add 1 cup of water to a bowl, then add 2 tsp of salt to the water. Stir until the salt is dissolved. Carefully pour this into the Salt Water jar. The eggshell should be completely covered in water.
- Add 1/2 cup of vinegar and 1/2 cup of water to a bowl. Stir the mixture, then pour it into the Vinegar jar. Be sure to completely cover the eggshell.
- Store the two jars in a safe location. You’ll want to keep them in a place where you can watch for any changes, but where the jar won’t accidentally be knocked over or disturbed.
- Now, it’s time to wait. It will take up to 24 hours to notice any differences in the eggshells.
- After 24 hours, observe the eggshells. Do you notice any difference between the eggshell pieces in the Salt Water and Vinegar jars? Gently touch the eggshells with a finger or blunt chopstick. Do the shells feel different?
- Leave the shells for another 24 hours and repeat your observations.
- Try leaving the shells even longer. Wait 24 more hours and check again: Have the eggshells changed even more?
Try this!
Try the same experiment with different amounts of vinegar, or with a different acidic liquid, such as lemon juice or orange juice. What happens if you submerge your eggshell in a solution with less vinegar—say, one part vinegar, two parts water? In comparison, what happens if you only use vinegar with no water at all?
You can also try this experiment with seashells from the craft or dollar store. How long does it take for a thin seashell to become fragile? What about a thicker one? Keep in mind that the shell will be damaged—don’t try this experiment with anything you’d like to keep!
How does it work?
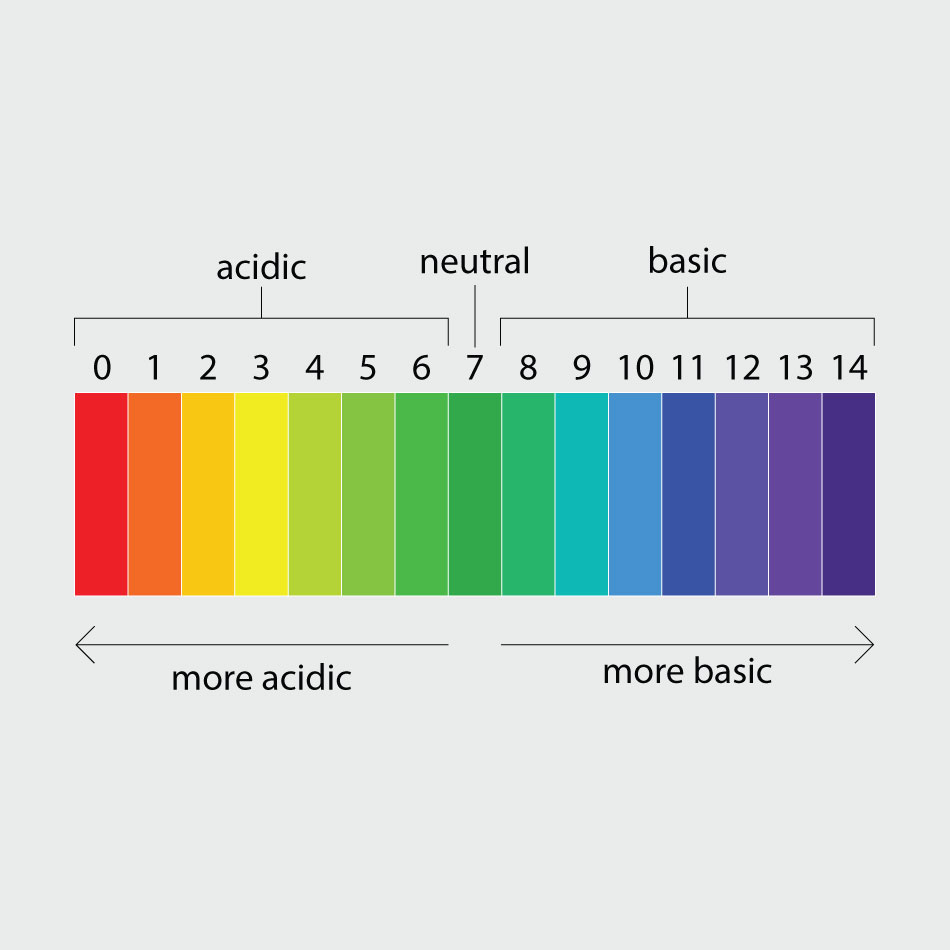
All solutions have a pH level, which represents how acidic or basic the liquid is. pH is measured on a scale. Think of the pH scale as a seesaw, with 7.0 falling in the middle. Any liquid with a pH of 7.0 is neutral. A pH of 0.0 to 7.0 means the liquid is acidic—the lower the number, the more acidic the solution. Meanwhile, a pH from 7.0 to 14.0 means the solution is basic. Another word for basic is alkaline.
Vinegar is very acidic with a pH of 2.4, plain water is neutral with a pH of 7.0 and salt water is slightly basic with a pH of 8.1. When you mixed the salt with the water, you created a slightly basic solution that had a pH and salt level similar to seawater. When you mixed vinegar with water, you created an acidic liquid.
It took time for the acidity to affect the eggshells, which are made of calcium carbonate. At first, the eggshells looked the same. Then, you probably noticed that the shell in the Vinegar jar started to float, and there were lots of bubbles on the surface. These bubbles were the result of a chemical reaction between the vinegar and the calcium carbonate in the eggshell, which created small amounts of carbon dioxide (CO2) gas.
As you observed, the eggshells in the Vinegar jar became weaker over time due to the acidity of the vinegar, and they began to dissolve. Meanwhile, the eggshell in the Salt Water jar stayed strong.

How does acidity affect marine life?
Just like the eggshells you used in your experiment, many animals in the ocean have shells made from calcium carbonate. Coral, crabs, mussels and other creatures use calcium from seawater to create their protective shells and skeletons.
Over time, our oceans are becoming more acidic due to human use of fossil fuels, which are energy sources like coal, petroleum oil and natural gas. When we burn fossil fuels to power our cars, homes and factories, large amounts of carbon dioxide gas are released into the atmosphere. This pollutes the air and eventually dissolves into the oceans, where it lowers the pH of the water.
Ocean acidity puts animals with shells and skeletons at risk, which has the potential to affect the entire ocean ecosystem. About 25% of all ocean species depend on coral reefs as their main habitat and food source. Without coral reef structures, many other species would become endangered or even extinct.
What can you do?
A carbon footprint is an estimate of how much carbon dioxide you generate as a result of your daily activities. By decreasing your use of fossil fuels and relying more on renewable energy sources, such as wind and solar energy, you can decrease your carbon footprint.
Here are a few things to try in your daily life:
- Walk or ride your bike to school instead of travelling in a car
- Eat less meat
- Plant a vegetable garden
- Reuse old items for new purposes whenever you can
What else can you do to decrease your carbon footprint? Write a list of ideas, then see how many you can try this week!